Nature May 2018: Abstract
Ling Zhang, Jake B. Bailey, Rohit H. Subramanian, Alexander Groisman, and F. Akif Tezcan
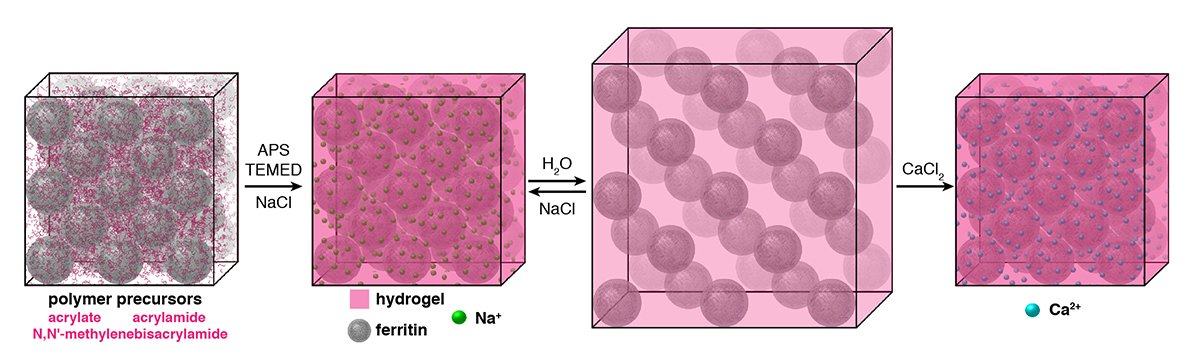
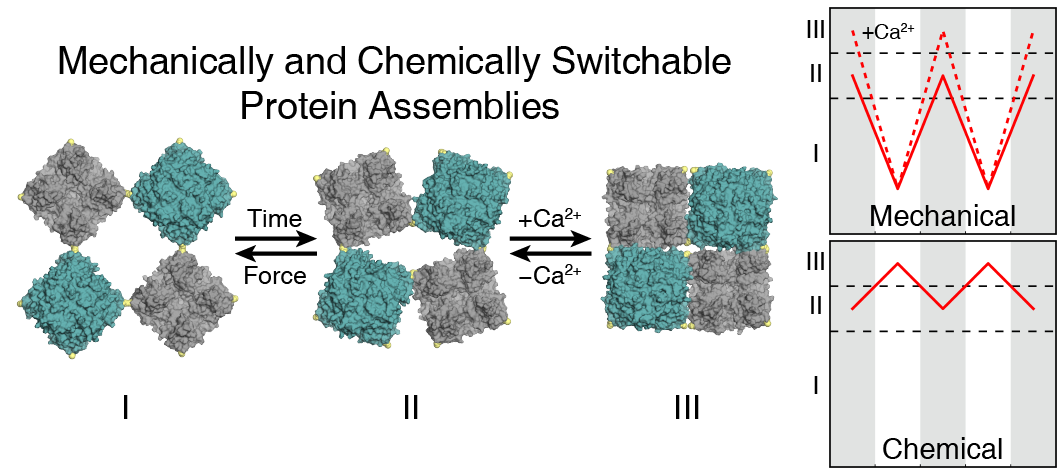
- The formation of condensed matter typically involves a trade-off between structural order and flexibility. As the extent and directionality of interactions between atomic or molecular components increase, materials generally become more ordered but less compliant, and vice versa. Nevertheless, high levels of structural order and flexibility are not necessarily mutually exclusive; there are many biological (such as microtubules, flagella, viruses) and synthetic assemblies (for example, dynamic molecular crystals and frameworks) that can undergo considerable structural transformations without losing their crystalline order and that have remarkable mechanical properties that are useful in diverse applications, such as selective sorption, separation, sensing and mechanoactuation. However, the extent of structural changes and the elasticity of such flexible crystals are constrained by the necessity to maintain a continuous network of bonding interactions between the constituents of the lattice. Consequently, even the most dynamic porous materials tend to be brittle and isolated as microcrystalline powders, whereas flexible organic or inorganic molecular crystals cannot expand without fracturing. Owing to their rigidity, crystalline materials rarely display self-healing behaviour. Here we report that macromolecular ferritin crystals with integrated hydrogel polymers can isotropically expand to 180 per cent of their original dimensions and more than 500 per cent of their original volume while retaining periodic order and faceted Wulff morphologies. Even after the separation of neighbouring ferritin molecules by 50 ångströms upon lattice expansion, specific molecular contacts between them can be reformed upon lattice contraction, resulting in the recovery of atomic-level periodicity and the highest-resolution ferritin structure reported so far. Dynamic bonding interactions between the hydrogel network and the ferritin molecules endow the crystals with the ability to resist fragmentation and self-heal efficiently, whereas the chemical tailorability of the ferritin molecules enables the creation of chemically and mechanically differentiated domains within single crystals.
This work was featured in a News & Views article and by Chemical & Engineering News (C&EN)! C&EN Article
Read the Behind the Paper post by Ling Zhang here!