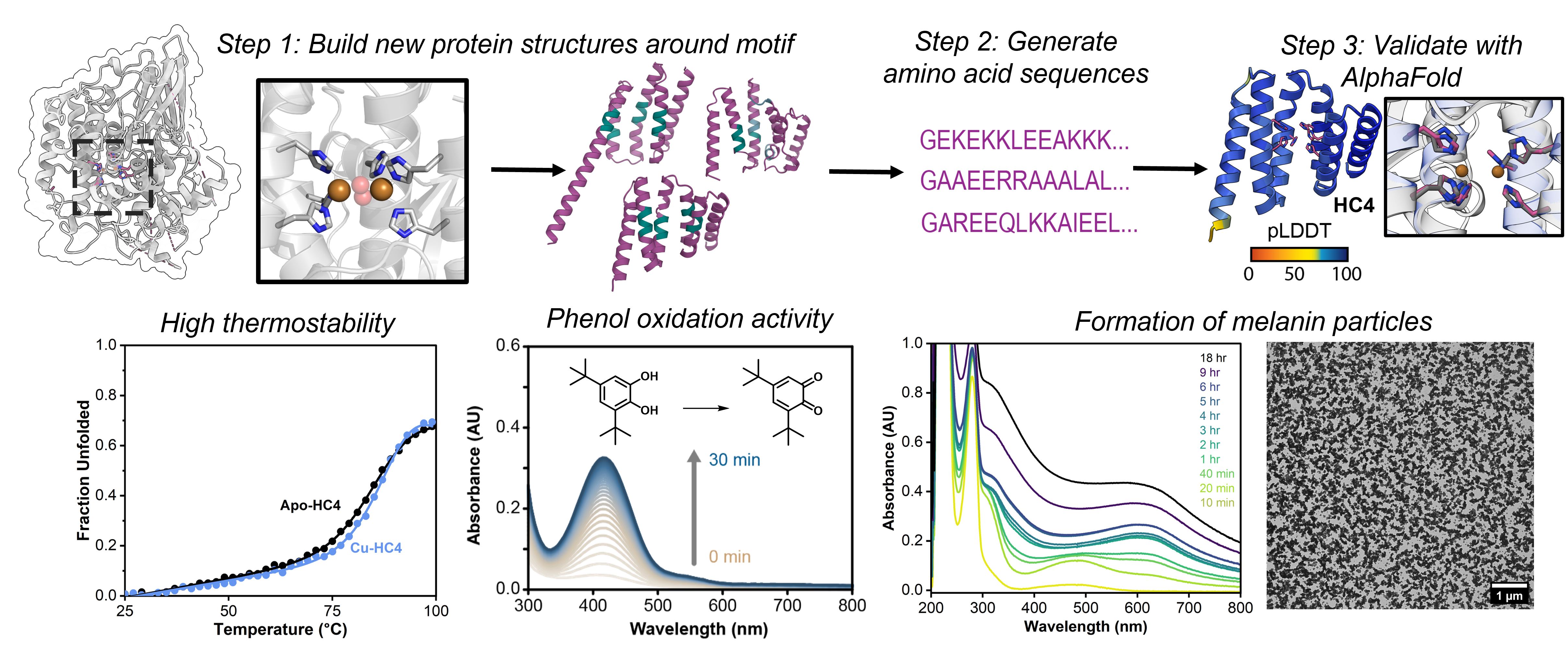
Metalloproteins carry out many cellular functions that are central to biology, ranging from photosynthetic water oxidation, nitrogen fixation and C-H/C-C bond activation to DNA synthesis/repair and cellular signaling. While our knowledge of how (metallo)proteins function has grown immensely thanks to advances in structure determination, spectroscopy and computation, we still possess only a superficial understanding of the interplay between protein structure/dynamics and metal coordination/reactivity. As a result, it has been challenging or even impossible (a) to predict the functional mechanism of metalloproteins simply by looking at their structures, (b) to emulate or improve upon the structures and functions of metalloproteins by design, and (c) to understand how complex bioinorganic functions may have emerged on simple peptide/protein scaffolds during natural evolution. The goal of the metalloprotein design subgroup is to address these three challenges by designing and constructing protein scaffolds with complex metal-based functions using a bottom-up approach.
Previously, our group developed distinct strategies (MeTIR and MASCoT), which use well-folded, stable proteins as building blocks to construct supramolecular assemblies with de novo designed properties.1-5 MeTIR and MASCoT have enabled the generation of metalloproteins with diverse functions such as in vivo active/evolvable esterase and β-lactamase activities, allosteric/selective metal binding, responsive protein cages, among others.6-12 More recently, we have begun to leverage the remarkable advances in AI- and machine-learning-based computational tools to design metalloproteins with atomic accuracy.13 The current focus of the metalloprotein design sub-group is to use and further develop these tools to construct de novo metalloenzymes that house complex metal centers with tunable/new-to-nature reactivities, can tolerate extreme operating conditions (high temperatures, organic solvents), and display substrate scopes beyond those of natural enzymes. Underlying all of these efforts is our desire to better understand how protein structure and dynamics control metal reactivity and to expand the inorganic chemistry of biology beyond the handful of transition metal ions and metal cofactors that natural evolution has selected.
——————————————————————————– Selected Publications ——————————————————————————–
1. Salgado, E. N., Radford, R. J. & Tezcan, F. A. Metal-Directed Protein Self-Assembly. Acc. Chem. Res. 43, 661-672 (2010).
2. Churchfield, L. A. & Tezcan, F. A. Design and Construction of Functional Supramolecular Metalloprotein Assemblies. Acc. Chem. Res. 52, 345-355 (2019).
3. Radford, R. J., Brodin, J. D., Salgado, E. N. & Tezcan, F. A. Expanding the utility of proteins as platforms for coordination chemistry. Coord. Chem. Rev. 255, 790-803 (2011).
4. Rittle, J., Field, M. J., Green, M. T. & Tezcan, F. A. An efficient, step-economical strategy for the design of functional metalloproteins. Nat. Chem. 11, 434-441 (2019).
5. Churchfield, Lewis A., George, A. & Tezcan, F. A. Repurposing proteins for new bioinorganic functions. Essays in Biochem. 61, 245-258 (2017).
6. Medina-Morales, A., Perez, A., Brodin, J. D. & Tezcan, F. A. In Vitro and Cellular Self-Assembly of a Zn-Binding Protein Cryptand via Templated Disulfide Bonds. J. Am. Chem. Soc. 135, 12013-12022 (2013).
7. Churchfield, L. A., Alberstein, R. G., Williamson, L. M. & Tezcan, F. A. Determining the Structural and Energetic Basis of Allostery in a De Novo Designed Metalloprotein Assembly. J. Am. Chem. Soc. 140, 10043-10053 (2018).
8. Churchfield, L. A., Medina-Morales, A., Brodin, J. D., Perez, A. & Tezcan, F. A. De Novo Design of an Allosteric Metalloprotein Assembly with Strained Disulfide Bonds. J. Am. Chem. Soc. 138, 13163-13166 (2016).
9. Song, W. J. & Tezcan, F. A. A designed supramolecular protein assembly with in vivo enzymatic activity. Science 346, 1525-1528 (2014).
11. Choi, T. S. & Tezcan, F. A. Design of a Flexible, Zn-Selective Protein Scaffold that Displays Anti-Irving–Williams Behavior. J. Am. Chem. Soc. 144, 18090-18100 (2022).
10. Song, W. J., Yu, J. & Tezcan, F. A. Importance of Scaffold Flexibility/Rigidity in the Design and Directed Evolution of Artificial Metallo-β-lactamases. J. Am. Chem. Soc. 139, 16772-16779 (2017).
12. Choi, T. S. & Tezcan, F. A. Design of a Flexible, Zn-Selective Protein Scaffold that Displays Anti-Irving–Williams Behavior. J. Am. Chem. Soc. 144, 18090-18100 (2022).
13. Hoffnagle, A. M. & Tezcan, F. A. Atomically Accurate Design of Metalloproteins with Predefined Coordination Geometries. J. Am. Chem. Soc. 145, 14208-14214 (2023).